Chemical equilibrium state
Features
The chemical equilibrium state has reverse, et al, moving, fixed, change, and six feature.
reverse: The object of chemical equilibrium studies is reversible.
etc: When balancing, the positive, inverse reaction rate is equal, that is, V is = V reverse.
Mobility: The reaction is still in progress, which is a dynamic balance, and the reaction is maximized.
Definitely: When the balance is achieved, the concentration of each component in the reaction mixture remains unchanged, the reaction rate remains unchanged, and the conversion of the reactants remains unchanged. The content of the division remains the same.
Change: The chemical balance is the same as all dynamic balances, it is conditional, temporary, relatively, when the conditions change, the balance state will be destroyed. The balance becomes unbalanced, and the new balance is established under new conditions, that is, the chemical balance occurs.
: The establishment and reaction of the chemical equilibrium state under certain conditions is independent. That is, whether it is from the positive reaction, or from the inverse reaction, or from any intermediate state, the effect is the same as the external condition is the same as the external condition.
Criterion
Gibbs free energy change:
When ΔRGM = 0, the reaction is maximized, in a balanced state. The establishment of chemical balance is premised in reversible reaction. The reversible reaction refers to a reaction that can be made forward and reverse direction under the same conditions. Most chemical reactions have reversibility and can be equilibrated to varying degrees.
From the behavior of the dynamics:
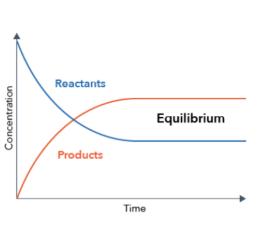
The reaction is started, the reaction concentration is large, the product concentration is small, so the normal reaction rate is greater than the reverse reaction rate. As the reaction progresses, the concentration of the reactant is constantly decreasing, the product concentration is increasing, so the normal reaction rate is constantly decreasing, and the inverse reaction rate is increasing. When positive, inverse reaction rates are equal, the concentration of each substance in the system no longer change, and the reaction reaches a balance.
Chemical balance is dynamic balance.
Learning to develop history
1940-1950
The basic law of thermodynamics has been clear, but some thermodynamic concepts are more vague, digital processing is very cumbersome, can't Used to solve a slightly complicated problem, such as the direction of the chemical reaction. At that time, most chemists were working on organic chemistry research, and some people tried to solve the direction of chemical reactions. This effort has other people trying to explore from other perspectives, some of which have proposed some empirical laws.
In this period, Danish Tom student and Beitro tried to explain the direction of chemical reactions from the thermal effect of chemical reactions. They believe that reaction heat is a measure of chemical affinity, and each simple or complex purification effect is accompanied by heat generation. Betelo has clearly expounded the same point of view, and called "maximum power principle". He believes that any purification of the impact of excessive energy is carried out towards the direction of the substance that releases the maximum energy. . Although he found some of the heat absorbing reaction, he can spontaneously assume it subjectively to the physical process of exothermic. This error has finally been recognized by him in the 1930s. At this time, he restricted the application range of "maximum principle" in the solid, and proposed the concept of "freedom" chemical heat .
1960-1980
Horsterman, Lexiate column and Fan Hof are also a certain contribution in this regard. First, Horsterman found in the sublimation of ammonium chloride found that in the thermal decomposition reaction, its decomposition pressure and temperature have a certain relationship, in line with the Claus, Clarion equation: DP / DT = Q / T (V'-V)
where Q represents the decomposition hot, V, V 'represents the total volume before and after decomposition. Fan Hof is based on the equation:
lnk = - (q / q) + c
This formula can be applied to any reaction Process, where Q represents the heat of absorption of the system (ie sublimation heat). Fan Hof called the above equilibration, and explained it. He said that any balance between the two different states of the substance is moved toward the balance direction of the two systems that produce heat. In 1874 and 1879, Moodye and Robin also put forward such a principle. Moodye proposed that the increase in pressure was conducive to the reaction of the volume correspondingly reduced. After this, Lexia Specially intended further universally explained this principle. He said that any system in the chemical balance, a change in one direction due to a variety of factors in the balance, which will lead to a conversion, and if this conversion is unique, then a kind of This factor varies the opposite change.
However, in this regard, it has been a prominent contribution to Gibbs, and his status in the history of thermochemistry is extremely important. Gibbs can summarize 4 aspects in power chemical contributions. First, On the basis of the second law established by Kulaus et al., Gibbs took out the balanced judgment, and the judgment of entropy was determined according to the correct limitations of the isolated system. Inside. Make general practical problems with universal processes. Second, is described in the internal energy, entropy, volume, and volume as a variable to the system state. It is pointed out that Tom is description of the status of Toms for use in the body. He advocated the status equation that the scientists were unfamiliar at the time, and in the three-dimensional coordinate diagram of internal, entropy and volume, there was a surface surface that completely describes all thermodynamic properties of the system. Third, Gibbs introduced "concentration" variable in thermodynamics, and clarifies the concentration of ingredients to the derivative of internal energy as "thermal learning". In this way, the thermodynamics can be used to handle multi-phase systems of multi-component, and the problems of chemical balance also have treated conditions. Fourth, he further discusses the balance problem under the influence of the system in electricity, magnetic and surface. Moreover, he exported that it is the simplest, most essentially the most abstract thermodynamic relationship, ie, interlaced, equilibrium state is the kind of state in which the law is zero.
Gibbs is mainly published in his three articles on balanced research results. In 1873, he successively published the first two from the Journal of the Connecticut College, immediately causing the attention of Maxwell. The first two texts in Gibbs can be said to be just a preparation, in 1876 and 1878, two parts have been published in the third article - "About the balance of the complex substance", the article is more than 300 pages, including more than 700 formulas. The first two articles are discussing a single chemical system system, which discusses the multi-group divided phase system. Due to the introduction of the thermal level, the problem of multi-component system can be processed as long as the single-component system state equation is slightly changed.
for Gibbs in the 1890s, Lexia Specially considers that this is a new field, and its importance can compare with the quality of quality. However, after the three articles of Gibs, the significance did not be recognized by most scientists, until 1891 was translated into German by Ostervad, 1899 Lexia Spert translated into a French publishing After that, the situation has changed. After Gibs, thermodynamics can only handle the ideal system. At this time, American Lovis published an article in 1901 and 1907, and proposed "Yi" and "activity" concept. Louis talks about " escape trend " this concept, pointing out some of the thermodynamics, such as temperature, pressure, concentration, thermal learning, etc., is the scale of the escape trend.
Louis's concept of escape and activity makes Gibbs' theory and develops, so that people may unify the deviation of the ideal system, so that the actual system is The form has the same thermodynamic relationship with the ideal system.
The symbol of the chemical equilibrium can be summarized as "first-class five unchanged", which is currently based on MA (G) + NB (g) == Pc (g) + Qd (g) as an example, the abstraction To specifically, improve students' understanding of this logo. 1. The first "first", that is, the positive reaction rate is equal to the reverse reaction rate, which means that the same reactant (or gene) in the reaction system rather than different substances in the same reaction. If different substances in the same reaction, the positive reaction rate and the inverse reaction rate must be required, the ratio of the two rates reverse (no one-way rate) and the ratio of the two rates is equal to the ratio of their corresponding chemometrics. In the test questions, there may be the following specific forms: (1) The positive response rate of the same substance is equal to the reverse reaction rate, such as A (consuming) = A (generated) or d (consumable) = d (generated). (2) The ratio of the positive response rate of a reactant and the ratio of the reverse reaction rate of the other reactant is equal to the ratio of the chemometrics, such as A (consumption): B (generated) = m: n, or c (consumption): d (generated) = p: q. (3) The ratio of the positive reaction rate of a reactant and the ratio of the reverse reaction rate of a product is equal to the ratio of the chemometrics, such as A (consumption): c (consumption) = m: p, or B (generated): d (generated) = N: Q. ⑷⑷ Take the same substance, the amount of the substance of the fracture chemical bond is equal to the amount of the substance forming the chemical bond. 2. The five unchanged "five unchangements", that is, the concentration of each component in the reaction mixture remains unchanged, and its significance means that the amount of the substance of each component is constant; the concentration of each component is constant; the percentage of each component is Not changed; the conversion rate of the reactants is constant; for reversible reactions all gas, when M + N╪P + Q, the amount of total substance of the mixed gas is unchanged. There may be the following specific forms in the test: (1) The amount of the substance of each component is constant, such as a certain temperature in a closed container, the number of molecules of A, B, C, and D will no longer change. (2) The concentration of each component is unchanged. When the external conditions are not changed, the color of the mixed gas does not change over time for the reversible reaction of the color substance. (3) The percentage content of each component is unchanged, such as the volume fraction of each component, the amount of material, and the mass fraction remain unchanged. The conversion rate of the reactant is unchanged, as under certain conditions, the conversion rate of A or B will no longer change. The above are applicable to the reversible reaction of the gas stoichiometric number before and after the reaction, but also an inverse reaction of the gas stoichiometry before and after the reaction. ⑸ For the reversible reaction of the pre-post-retrieval metallometric change in the gases, the amount of the total substance of the mixed gas is unchanged. When M + N╪P + Q, when constant temperature constant, the total pressure of the system does not change; when M + N╪P + Q, the total volume of the system does not change when the constant temperature is constant; when M + When N╪P + Q, the average relative molecular mass of the mixed gas does not change during constantre. The special mark of the dual chemical equilibrium is under constant reliability conditions, whether the density of the mixed gas can be used as a marker of the chemical equilibrium, which is mainly related to non-gas-free substances in reversible reactions. Because the gas density under this condition is only related to the quality of the gas, if it is the reversible reaction of the gas-state substance, under constant relocation conditions, whether or not the balance is achieved, the total mass of the mixed gas does not change, the density of the mixed gas does not occur Variety, it is not possible to be a judgment mark in a chemical equilibrium state. If there is a reversible reaction involved in non-gaseous substances, under constant reliability conditions, only the total mass of the mixed gas does not change, the density of the mixed gas does not change, at which time density can be used as a chemical equilibrium state. Judgment flag.
Latest: First class capital