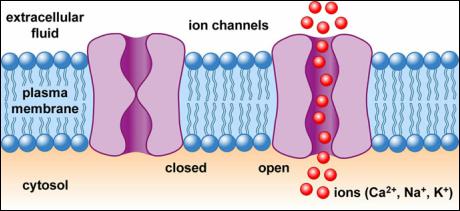
Cell membrane ion channel
Proposalprocess
In1998,McKinnonmadeahigh-resolutionthree-dimensionalstructureimageoftheionchannelproteinKcsAofStreptomyces,andforthefirsttimeunderstoodtheroleofionchannelsfromtheatomiclevelWay.Thereisakindof"filter"intheKcsAionchannelthatallowspotassiumions(K+)topass,butdoesnotallowthesmallersodiumions(Na+)ofthesamefamilyofelementstopass,whichpuzzlesscientists.However,basedonthethree-dimensionalstructureofKcsA,McKinnonfoundthatthepositionsofthefouroxygenatomsonthesideofthe"filter"intheionchannelareexactlythesameaspotassiumionsinanaqueoussolution,thatis,theoxygenandwatermoleculesonthesideofthefilter.Theoxygendistanceisthesame,sopotassiumionscanpassthroughthechannelsafely,justlikeinwater;butsodiumionsaresmallinsizeandcannotsmoothlyconnecttothefouroxygenatomsonthesideofthefilter,sotheycanonlystayintheaqueoussolution,noteasilyGothroughthechannel.Theopeningandclosingofionchannelsiscontrolledbycells.McKinnondiscoveredthatthereisagateatthebottomoftheionchannel.Whentheionchannelreceivesaspecificsignal,theproteinstructureoftheionchannelwillchange,causingthegatetoopenandclose.
Mackinnon’sresearchonthestructureandmechanismofpotassiumionchannelsisabreakthroughinbiochemistry,biophysicsandotherfields.Italsoguidesthedevelopmentofnewdrugsforneurologicaldiseases,muscleandheartdiseases.Anewdirection.
Abriefhistoryofresearch
Inthestudyofthemechanismofbioelectricitygeneration,changesinthepermeabilityofbiofilmstoionshavebeendiscovered.In1902,J.Bernsteinproposedinhismembranetheorythatnervecellmembraneshaveselectivepermeabilitytopotassiumions.In1939,A.L.HodgkinandA.F.Huxleyinsertedmicroelectrodesintothegiantnervefibersofsquid,anddirectlymeasuredthepotentialdifferencebetweentheinsideandoutsideofthemembrane.In1949,ALHodgkinandB.Katzproposedthemembranepotentialionhypothesisbasedonaseriesofwork.Theybelievedthattheactionpotentialofthecellmembranewascausedbyarapidandspecificincreaseinthepermeabilityofthemembranetosodiumions,whichwascalledthe"sodiumtheory".Particularlyimportantisthatin1952,ALHodgkinandAFHuxleyusedvoltageclamptechnologytocarryoutadetailedquantitativestudyontheioniccurrentandconductanceofthecellmembraneonthesquidgiantnerveaxon.TheresultsshowedthatthecurrentsofNa+andK+werecombinedConductanceisafunctionofmembranepotentialandtime,andtheconceptofionchannelswasfirstproposed.Theirmodel(H-Hmodel)believesthattheK+channelofthecellmembraneiscontrolledbyfourchargedparticlesonthemembrane.Whenthefourparticlesmovetoacertainpositionundertheactionofthemembraneelectricfield,K+canpassthroughthemembrane.Ontheotherhand,in1955,CastroandB.Katz'sresearchonthesynaptictransmissionprocessofthenerve-musclejunctionfoundthatthepost-synapticmembraneendplatepotentialoccursbecausetheneurotransmitteracetylcholine(Ach)actsonTheresultsofthereceptorsontheendplatemembrane,thusconfirmingthechannelsregulatedbychemicaltransmitters.Inthe1960s,studiesonthepermeabilityofdifferentionswithvariousbiologicalmaterialsshowedthatvariousionshadtheirownspecifictransportmechanismsonthemembrane.Itwasonceproposedthattransportmechanismsweremodelsofcarriers,holes,andionexchange.In1973and1974,CMArmstrong,F.Besanilla,RDKeynes,andE.Rogersmeasuredthemovementoftheintra-membranechargerelatedtotheopeningofionchannelsontheaxons,whichiscalledgating.Electriccurrentconfirmsthecompliancebetweentheopeningofionchannelsandthemovementofchargedcomponentsinthemembrane.In1976,E.NellandB.Sackmanfoundedtheionsingle-channelcurrentrecordingtechnology,andithasbeenquicklypromotedandapplied.Inrecentyears,somenewionchannelshavebeendiscoveredusingthistechnology,whichprovidesapowerfulforin-depthstudyofthestructureandfunctionofthechannel.Toolof.Intheearly1980s,scholarssuccessivelyseparatedandpurifiedsomefunctionalproteinsthattransportionsfromthecellmembrane,andsuccessfullyreconstructedthechannelfunctionontheartificialmembrane,thusconfirmingthattheionchannelentityissomespecialproteinmoleculesortheircompoundonthemembrane.Things.Inrecentyears,scientistshaveappliedgenerecombinationtechnologytostudythestructureofionchannels.In1982and1984,NumerandhiscollaboratorssuccessivelydeterminedtheaminoacidsequencesofN-typeAchreceptorandNa+channelprotein.
Researchmethods
Thestudyofionchannelstructureandfunctionrequirescomprehensiveapplicationofvarioustechnologies,including:voltageandcurrentclampingtechnology,single-channelcurrentrecordingtechnology,channelproteinseparation,andpurificationBiochemicaltechnology,artificialmembraneionchannelreconstructiontechnology,channelpharmacology,generecombinationtechnologyandsomephysicalandchemicaltechnologies.
Basicfeatures
Ionchannelscanbedividedintotwocategoriesaccordingtotheiractivationmethods:oneisvoltage-activatedchannels,thatis,theopeningofthechanneliscontrolledbythemembranepotential,suchasNa+,Ca+,Cl-andsometypesofK+channels;theotherischemical-activatedchannels,thatis,channelsthatareactivatedbytheinteractionofchemicalswithreceptorsonthemembrane,suchasAchreceptorchannels,aminoacidreceptorchannels,andCa+ActivatedK+channels,etc.1.Selectivity:Referstothecharacteristicthatachannelpreferentiallyallowscertainionstopassthrough,whileotherionsarenoteasytopassthroughthechannel.Forexample,whenthesodiumchannelisopen,sodiumionscanpass,butpotassiumionscannotpass.2.Switching:Therearetwostatesofionchannels,namelyopenandclosedstates.Inmostcases,ionchannelsareclosedandonlyopenundercertainconditions.Theprocessofturningachannelfromaclosedstatetoanopenstateiscalledactivation,andtheprocessofturningachannelfromanopenstatetoaclosedstateiscalledinactivation.Theopeningandactivationprocessofthechannelhasacertainrate,usuallyveryfast,calculatedinmilliseconds(ms).
Channelclassification
Theopeningandclosingofionchannelsiscalledgating.Accordingtodifferentgatingmechanisms,ionchannelsaredividedintothreecategories:⑴Voltagegating,alsoknownasvoltage-dependentorvoltage-sensitiveionchannels:theyareopenedandclosedduetochangesinmembranepotential,andarenamedafterthemosteasilypassedion.Therearefourmaintypesofpotassium,sodium,calcium,andchloridechannels,eachofwhichisdividedintoseveralsubtypes.(2)Ligand-gated,alsoknownaschemical-gatedionchannel,isopenedbythebindingsiteofthetransmitterandthechannelproteinreceptormolecule,namedafterthetransmitterreceptor,suchasacetylcholinereceptorchannel,glutamatereceptorChannels,aspartatereceptorchannelsandothernon-selectivecationchannelsareopenedbyligandsactingonthecorrespondingreceptors,whileallowingsodium,calciumorpotassiumtopassthrough.⑶Mechanicallygated,alsoknownasmechanicallysensitiveionchannels,arechannelsthatsensechangesincellmembranesurfacestressandrealizethetransductionofextracellularmechanicalsignalsintothecell.Accordingtotheirpermeability,theyaredividedintoion-selectiveandnon-ion-selectivechannels.Accordingtotheirfunction,theyaredividedintotension-activatedandtension-inactivatedionchannels.Inaddition,thereareorganelleionchannels,suchasvoltage-dependentanionchannelswidelydistributedontheoutermitochondrialmembraneofmammaliancells,thesarcoplasmicreticulumorendoplasmicreticulum,andreceptorchannelsandreceptorchannelsonthemembrane.Voltage-gatedcalciumchannels(VGC)aredividedintofoursubtypes:L-type(Long-lasting),N-type(No-Longlasting,non-tsansient),T-type(Transient)andP/Q.L-typechannel:conductanceLargersize,slowinactivation,longduration,andneedstrongdepolarizationtoactivate.Itisexpressedinvarioustissuessuchascardiovascular,endocrineandnerve,andparticipatesinelectrical-constrictioncouplingandregulationofmetabolism.T-channel:smallconductance,fastinactivation,weakdepolarizingcurrentcanbeactivated,itismainlydistributedintheheartandvascularsmoothmuscle,triggeringpacingelectricalactivity.N-typechannel:Inactivationisfasterandrequiresstrongdepolarizingcurrentactivation.Itisonlyfoundinnervetissueandmainlytriggersthereleaseofsympatheticneurotransmitters.P/Qchannels:Thesameα1subunits(α1A)arecollectivelyreferredtoasP/Qcalciumchannels.P/Qtypecalciumchannelsplayanimportantroleintheprocessofneurotransmitterrelease.Potassiumchannel:Aproteincomplexthatiswidelypresentonthecellmembranethroughwhichpotassiumionsselectivelypass,formingalargefamilyofchannelsinstructureandfunction.Potassiumionchannelscangenerallybedividedintofourbasictypes:voltage-gatedpotassiumchannels(Voltage-gatedK+Channels,KV),calcium-activatedpotassiumchannels(Calcium-activatedK+Channels,KCa),adenosinetriphosphate-sensitivepotassiumchannels(ATP-sensitive)K+Channels,KATP).Voltage-gatedpotassiumchannelsarefurtherdividedinto:InwardrectifierK+Channds(Kir),delayedoutwardrectifierpotassiumchannels,andtransientoutwardpotassiumchannels.
Latest: 11th century
Next: Pre-Qin